
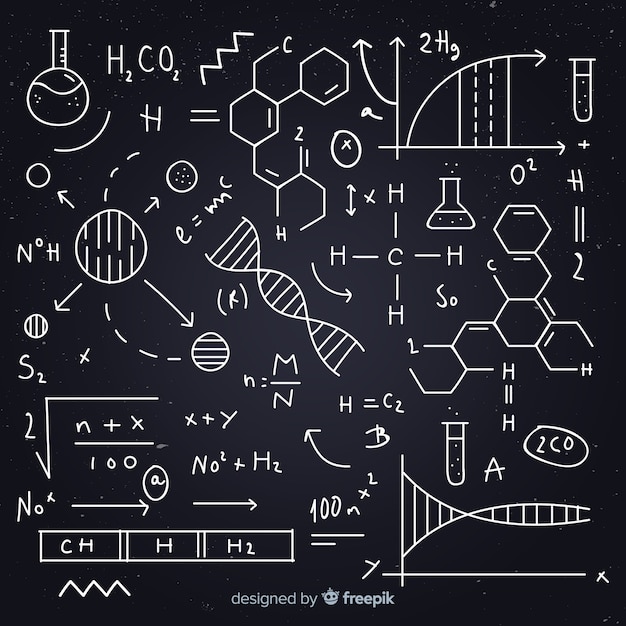
Using a chemical equation, we can also determine the equilibrium constant of the reaction (K eq). The balanced chemical equation for the above reaction is: As a result, it fails to correctly represent what takes place during the reaction.įor example, consider the example give below: If the numbers of each kind of atom on the two sides of an equation are different, then the reaction is unbalanced. That is because in a reaction, usually the reactants’ bonds are broken and new bonds are to give rise to the products. The only difference on each side of the equation is the arrangement of the atoms to form ions or molecules. Obeying the laws of conservation of mass and charge, both the numbers of every type of atom and the total charge have to be the same on both sides of a balanced chemical equation. In a chemical equation, the reactants and products are conventionally shown on the left and right sides of an arrow, respectively. What occurs during a chemical reaction is the rearrangement of atoms. According to the law of conservation of mass, atoms are neither created nor destroyed during a chemical reaction. In chemistry, an equation is basically the symbolic representation of a chemical reaction. What Is A Chemical Equation?īefore we read about the chemical equation calculator, let us first understand what a chemical equation is. Let’s take a closer look at the features of this unique chemical equation balancer & calculator. You can rely on it to give you fast and accurate results. If you have been facing difficulties with the same, or are simply short of time, you could try using the chemical equation calculator for balancing the equation. These equations need to be balanced in order to obey the laws of conservation of mass and energy.īalancing a chemical equation can be a rather tricky and time-consuming task at times. For example, chemical equations are used to represent how a chemical reaction takes place. Thus, one needs to be strong in those areas to understand chemical reactions and properties.

The foundations of chemistry are based on mathematics and logic. Isn’t it fascinating to think how there are millions of chemical reactions taking place in our body as we run, sit, eat, or even just breathe? The knowledge of chemistry is thus essential for students aiming to become doctors, pharmacists, physicists, nutritionists, geologists, or agronomists. It has become even more relevant today because of the demand for eco-friendly products and procedures. In our everyday life, chemistry plays a vital role in areas like cleaning, cooking, and medicine. The laws of chemistry dictate many of the natural phenomena we see occurring around us – be it the blazing sun above, clouds, volcanic eruptions, photosynthesis, and even our own bodies. Chemistry is highly practical, logical, and valuable in all aspects of our life in some way or the other. Although it is often considered a complicated and monotonous discipline, the reality is much different. Visit the go-to place for formulae of various concepts on and learn the concepts effortlessly.Chemistry is one of the most important branches of natural science. Decrease of pressure (from less moles to more moles)Ĩ.Increase of pressure (from more moles to less moles).


 0 kommentar(er)
0 kommentar(er)
